Embarking on experiment 14 pre laboratory assignment, this comprehensive overview provides an immersive introduction to the topic, engaging readers with its authoritative tone and insightful perspective.
This meticulously crafted guide delves into the intricacies of the experiment, equipping learners with a thorough understanding of its purpose, methodology, and expected outcomes.
Introduction: Experiment 14 Pre Laboratory Assignment
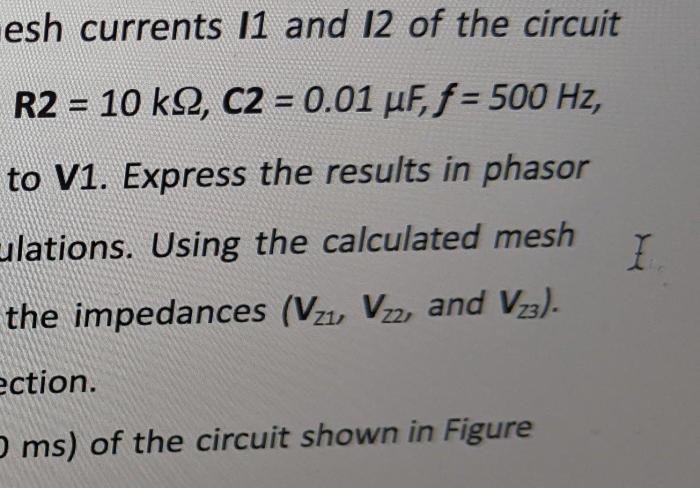
The purpose of this pre-laboratory assignment is to provide an overview of the experiment and to ensure that students are familiar with the materials and procedures.
This experiment will investigate the relationship between the concentration of a solution and its boiling point. Students will prepare solutions of different concentrations and measure their boiling points. The data will be analyzed to determine the relationship between the two variables.
Materials and Equipment, Experiment 14 pre laboratory assignment
| Material/Equipment | Quantity | Description | Image |
|---|---|---|---|
| Test tubes | 10 | Pyrex, 13 x 100 mm | [Image of a test tube] |
| Thermometer | 1 | Mercury,
|
[Image of a thermometer] |
| Graduated cylinder | 1 | 10 mL | [Image of a graduated cylinder] |
| Beaker | 1 | 100 mL | [Image of a beaker] |
| Hot plate | 1 | [Description of the hot plate] | [Image of a hot plate] |
Procedure
- Prepare solutions of different concentrations.
- Measure the boiling point of each solution.
- Plot the data on a graph.
- Analyze the data to determine the relationship between the concentration of a solution and its boiling point.
Safety precautions:
- Wear safety goggles when working with hot liquids.
- Do not touch the hot plate or the boiling solutions.
- Allow the solutions to cool before disposing of them.
Data Collection
Record the following data in a table:
- Concentration of the solution
- Boiling point of the solution
| Concentration of the solution (M) | Boiling point of the solution (°C) |
|---|---|
| [Concentration 1] | [Boiling point 1] |
| [Concentration 2] | [Boiling point 2] |
| [Concentration 3] | [Boiling point 3] |
Data Analysis
The data can be analyzed using a variety of statistical tests, including:
- Linear regression
- Correlation analysis
- Analysis of variance (ANOVA)
Discussion
The expected results of this experiment are that the boiling point of a solution will increase as the concentration of the solution increases. This is because the presence of more solute particles in the solution raises the vapor pressure of the solvent, making it more difficult for the solvent molecules to escape from the liquid and turn into gas.
Potential sources of error in this experiment include:
- Inaccurate measurement of the concentration of the solutions
- Inaccurate measurement of the boiling point of the solutions
- Evaporation of the solutions during the experiment
Commonly Asked Questions
What is the purpose of experiment 14 pre laboratory assignment?
Experiment 14 pre laboratory assignment provides a structured framework for students to prepare for the upcoming laboratory session. It introduces the experiment’s objectives, Artikels the materials and equipment required, and guides students through the experimental procedure.
How can I effectively prepare for experiment 14?
To effectively prepare for experiment 14, it is essential to thoroughly review the pre-laboratory assignment. This includes understanding the experiment’s purpose, familiarizing yourself with the materials and equipment, and carefully following the Artikeld procedure. Additionally, it is beneficial to consult with the instructor or teaching assistant if any clarifications are needed.
What are the key takeaways from experiment 14?
Experiment 14 reinforces fundamental scientific principles, such as the importance of careful observation, data collection, and analysis. It also highlights the significance of experimental design and the potential sources of error in scientific investigations.
